Hepatitis B infection
Author: Dr Tony Williams FFOM, Consultant Occupational Physician, Working Fit Ltd
Date: 28th November 2015.
Hepatitis B is caused by a Hepadnavirus which is extremely resistant to temperature and humidity. It can survive for 15 years at -20oC, six months at room temperature and seven days at 44oC. The virus contains a loop of DNA (HBe) with DNA polymerase to assist with replication, surrounded by core protein (HBc) with surface protein outside this (HBs).
Risk of infection with Hepatitis B
Transmission of infection is easy; the virus is highly infective, much more so than Hepatitis C or HIV. Worldwide one of the most common routes of transmission is maternal-fetal, probably during childbirth. The next most common is sexual transmission, while intravenous drug abuse is a common route in UK. There are numerous examples of transmission from patients to healthcare workers and from healthcare workers from patients. Infection can only result from whole virus particules, not just isolated antigens.
An orienteer contracted Hepatitis B from a scratch on a thorn which had just scratched another runner who was Hepatitis B positive (Ringerts 1971).
Hepatitis B viral antigens
The Hepatitis B virus has three key antigens. The surface antigen, HBsAg, can be reproduced and secreted alone without the remaining parts of the virus. The core antigen, HBcAg, is a marker of infection. The core gene antigen HBeAg is a marker of active viral replication.
A variant of Hepatitis B does not produce the standard HBeAg, so is called ‘HBeAg negative’, but it still replicates DNA and is infective. This is found in around 50% of patients from Asia and Africa, and around 10-30% of patients from USA and Europe.
Infection can only result from complete functioning viruses. Individuals who produce only part of a virus are not infective.
Typical infection viral life cycle
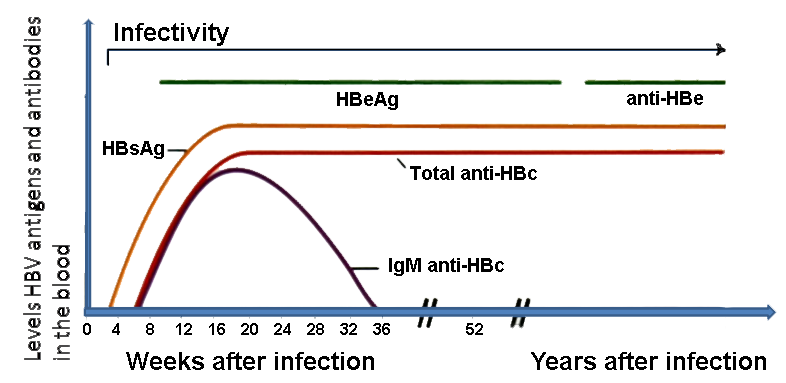
Following injection there is an incubation period of 45-200 days, usually around 75 days, with no immune response. An immune reaction to the virus then begins as virus particles are released with HBeAg seen in serum and a declining level of HBV DNA in serum. During this period of 3-4 weeks the patient may be symptomatic although some show no symptoms. Five percent of adults do not show a typical immune response and go on to develop chronic viral hepatitis.
Typically after this period of immune response, viral replication ceases. Levels of HBeAg and HBsAg are still detected but HBV DNA is no longer detected. Antigen levels then gradually decline, with a rise in antibodies to all viral antigens.
When patients progress to chronic infection, HBsAg continues to be produced along with HBeAg and HBV DNA. The antigens are markers of continued infection while HBV DNA is a measure of infectivity.
Acute infection response
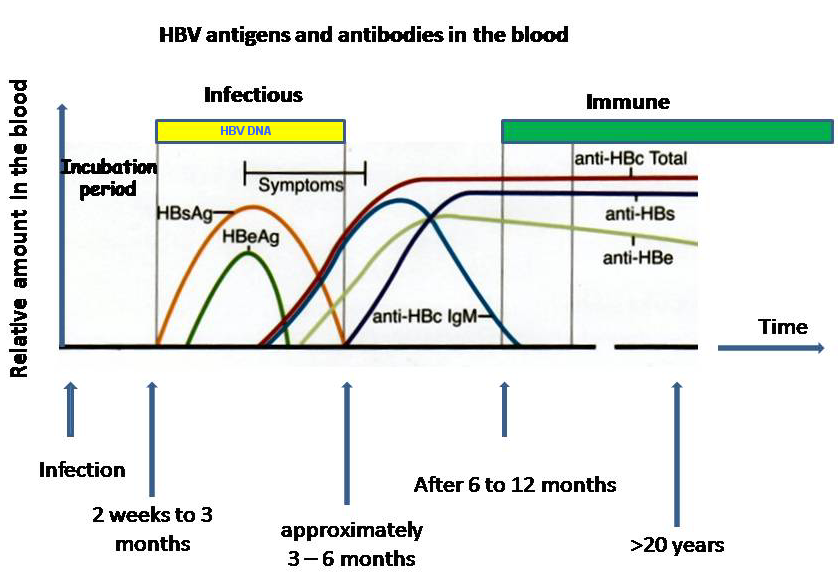
Chronic infection blood markers
Testing for infection
- HBsAg is a marker of current infection
- Anti-HBs is a marker of immunity
- Anti-HBc is a marker of previous exposure or chronic infection
- Anti-HBc IgM is a marker of recent exposure or current infectivity
- HBeAg is a marker of high viral levels
- Anti-HBe is a marker of clearance and immunity
- Hep B viral DNA level is amarker of infectivity
Risks from Hepatitis B
The main risk is acute hepatitis as infected hepatocytes are destroyed by the immune system. If the immune system is impaired, chronic infection may result. This is only seen in around 5% of adults, but is much more common in children (50%) and neonates (90%) (Te et al, 2010).
Chronic infection can also be accompanied by chronic hepatitis. This can progress to cirrhosis, and around 9% of those with cirrhosis develop hepatocellular carcinoma.
Treatment of Hepatitis B
Early treatment with immunoglobulin should successfully prevent initial infection with the virus. For those who have already developed chronic infection, antiviral therapy with pegylated interferon alpha (PEG-IFN-α), entecavir (ETV), and tenofovir disoproxil fumarate (TDF) can result in clearance of infection in around a third of patients. Other treatments are in development. Once patients have developed end-stage disease with cirrhosis, the only effective treatment is liver transplant.
Health care workers
The primary policy for health care workers is immunisation. All health care workers shoud be offered immunisation against Hep B, but immunisation is not a substitute for good infection control practices. All staff performing EPPs or working in renal dialysis units must demonstrate immunity or freedom from infection with an IVS sample.
Immunisation
The vaccine is highly effective in those who respond. A low response rate is associated with older age, obesity, smoking and immunosuppresion, and some are non-responders. The vaccine is a recombinant Hep B surface antigen produced in yeast. It is completely safe and there is no significant risk of side effects. It is given in a course of three injections, and when immunising after exposure the first injection is accompanied by immunoglobulin. Ninety-five percent of those immunised achieve an HBsAb level greater than 10M IU/ml. Of those who fail to respond to one course, 25-50% respond to one additional dose, and 50-75% respond to a second 3-dose course. Studies also suggest a third course injected intradermally raises response rates further.
Immunisation protocols (see the Green Book)
- Standard course: 0, 1 and 6 months, check response 2 months after 3rd dose.
- Accelerated course: 0, 1, 2 and 12 months, check response 2 months after 3rd dose but must still give 4th dose.
- Defaulters must restart course
Non-responders
Should be counselled by OH to immediately request support with immunoglobulin after exposure and sharps injuries, and be checked annually for HBsAg.
Hepatitis B positive health care workers undertaking EPP (Department of Health, 2012)
- A health care worker cannot undertake EPPs if they are HBeAg positive
- All testing must be on IVS samples sent to a designated laboratory.
- They can do EPPs if they are HBeAg negative and have a viral load less than 103 genome equivalents/ml.
- If they have a viral load greater than 103 genome equivalents/ml, they can do EPP if one year after treatment has finished their viral load is less than103 genome equivalents/ml. The viral load should be monitored at six months and then annually.
- If they have a viral load greater than 103 genome equivalents/ml but less than 105 genome equivalents/ml, and are on antiviral treatment, they can undertake EPP if their viral load reduces to less than 103 genome equivalents/ml on two consecutive tests one month apart. Viral load must be monitored every three months, and they must be monitored by a consultant occupational physician. They should be referred to a hepatologist if the viral load rises above 103 genome equivalents/ml. If they stop antivirals they must stop EPP immediately. If patients are accidentally exposed, they must be risk assessed and may need PEP.
DEPARTMENT OF HEALTH. Hepatitis B infected healthcare workers and antiviral therapy. 2012.
RINGERTZ O. Serum hepatitis in Swedish track finders. Scand. J. Infect. Dis. 1971; 2 (Suppl. 2): 3–25.
TE HS, JENSEN DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010 Feb. 14(1):1-21, vii.